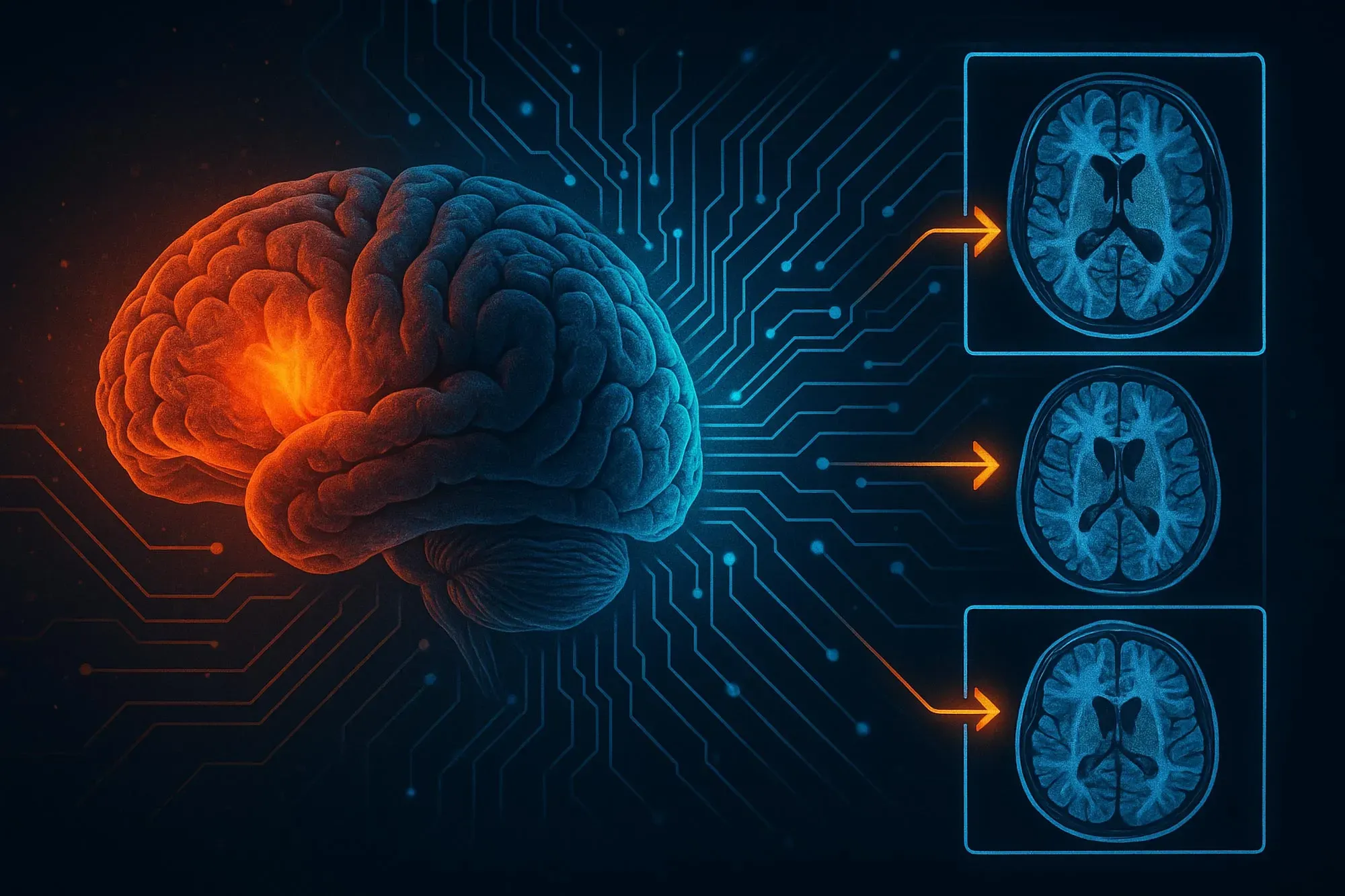
Summary: A team of scientists has created an advanced AI system capable of forecasting the return of gliomas in children by examining a series of post‑treatment brain scans. Instead of relying on a single MRI, the approach—known as temporal learning—tracks and interprets minute, progressive changes across multiple images taken over time, allowing for far more precise predictions of tumor recurrence.
The study shows that using multiple images significantly improves traditional single-scan analysis, with up to 89 percent accuracy. This approach could reduce the burden of repeated scans or enable earlier interventions in the future.
Important facts:
- Benefit of temporary learning: An AI trained on multiple post-treatment MRIs predicted recurrence with 89% accuracy.
- Effective images: Just 4 to 6 scans were enough to solidify the AI’s predictive ability.
- Medical capacity: This could reduce the frequency of screening in low-risk patients or allow higher-risk patients to be treated earlier.
Source: Mass General
Artificial intelligence (AI) offers great potential to analyze large amounts of medical image data and detect patterns that human observers may miss.
AI-assisted interpretation of brain scans could improve care for children with brain tumors, also known as gliomas. These tumors are usually treatable, but the risk of recurrence varies. Researchers at Mass General Brigham and Women’s Hospital and Dana-Farber/Boston Children’s Cancer and Blood Disorders Center have trained a deep learning algorithm to analyze consecutive brain scans after treatment and identify patients at risk of cancer recurrence.
“Many childhood gliomas can be cured with surgery alone, but recurrence can have devastating consequences,” said corresponding author Dr. Benjamin Kahn, of the Artificial Intelligence in Medicine (AIM) program at Mass. General Brigham.
It is very difficult to predict who is at risk of relapse. Therefore, patients are monitored regularly for years using MRI. This can be stressful and burdensome for children and their families. We need better tools to identify early which patients are at high risk of relapse. Research into relatively rare diseases, such as childhood cancer, can be hampered by a lack of data. The study, supported by the National Institutes of Health, leveraged institutional collaborations across the country to collect nearly 4,000 MRI scans from 715 pediatric patients.
To enhance the AI’s ability to detect recurrence, the team applied a method known as temporal learning—training the model to integrate and interpret a series of post‑surgery brain scans taken over several months, rather than relying on a single image. This approach allows the system to recognize subtle, progressive changes that may signal a tumor’s return. AI models for medical imaging are typically trained to draw conclusions from individual scans. Temporal learning, which has not yet been used in AI research in medical imaging, incorporates images acquired over time into the algorithm’s prediction of cancer recurrence.
To develop the temporal learning model, the researchers first trained the model to sort the patient’s postoperative MRI scans chronologically. This allowed the model to learn to detect subtle changes.
The researchers then modified the model to accurately account for post-cancer changes, if applicable. The researchers ultimately found that their temporal learning model could predict the recurrence of low‑ or high‑grade gliomas one year after treatment with an accuracy of 75% to 89%, a substantial improvement over predictions based on single MRI images, which hovered around 50%, essentially no better than chance.
By providing the AI with images from multiple time points after treatment, the model’s predictive accuracy increased. However, only four to six images were needed before this level of improvement was reached.
The researchers emphasize that further validation is needed in other settings before the product can be applied clinically. Ultimately, they hope to launch clinical trials to see if AI-based risk predictions can lead to improvements in care, by reducing the frequency of imaging in low-risk patients or pre-treating high-risk patients with specific additional treatments.
“We have demonstrated that AI can successfully interpret and generate predictions from sequences of images, rather than relying on single scans,” said Divyanshu Tak, principal investigator of the Artificial Intelligence in Medicine (AIM) Program at Brigham and Women’s Hospital’s Department of Radiation Oncology.
“This technique has potential applications in many situations where patients undergo serial and longitudinal imaging, and we are excited to see what this project will bring us.”
Authorship: In addition to Kan and Taak, the authors of Massa Brigham General include Benjamin A. Garumsa, Anna Zapishchekova, Zhizhong Ye, Maryam Mahutiha, Tafadzwa Chonzwa, Hugo J.W.L. Ertz, and Daphne Haas Kogan. Other authors include Sridhar Vijayapyam, Juan Carlos Clement Pardo, Celia Smith, Ariana M. Familiar, Kevin
Funding: The study received funding from the National Institutes of Health/National Cancer Institute (NIH/NCI) under grants U54 CA274516 and P50 CA165962, as well as support from the Botha‑Chan Low‑Grade Glioma Consortium. The researchers also acknowledged the Childhood Brain Tumor Network (CBTN) for providing access to valuable clinical and imaging data.
Abstract
Longitudinal prediction of pediatric glioma risk using temporal deep learning.
Background
The patterns and severity of recurrence are variable and difficult to predict using established clinical and genomic markers. Therefore, almost all children, regardless of their individual risk of recurrence, undergo regular and long-term brain imaging (MRI). Longitudinal deep learning analysis of serial MRI scans may be an effective approach to improve the prediction of individual recurrence in gliomas and other cancers. However, progress is limited by data availability and current machine learning methods.
Methods
We have developed a self-supervised temporal deep learning method specifically designed for longitudinal medical image analysis. A multi-stage model encodes serial MRI scans of patients and is trained to classify correct chronological order as a predicate task.
The pre-trained model is then fine-tuned using the patient’s historical post-surgical surveillance scans to predict the recurrence of pediatric glioma at the primary point of interest (in this case, within one year of the last scan). We applied the model to 3994 scans of 715 patients treated at three different institutions in the context of low- and high-grade gliomas in children.
Results
Using longitudinal image analysis with temporal learning significantly boosted recurrence prediction accuracy, improving the F1 score by up to 58.5% (range: 6.6%–58.5%) compared with traditional methods across all datasets. Gains were observed in both low‑ and high‑grade gliomas, along with a notable improvement in area under the curve (AUC) across all ROC analyses. Prediction accuracy increased steadily as more historical scans per patient were available, with optimal performance reached after three to six scans, depending on the dataset.
Conclusions
Temporal deep learning enables high‑throughput, longitudinal medical image analysis, enhancing prognostic decision‑making in pediatric brain tumors. This approach has broad potential for monitoring and risk prediction in patients with other cancers and chronic diseases that require ongoing surveillance imaging.